Clinical Study & Trial Support
UKBS provides end-to-end support for clinical research studies and clinical trials.
From site logistics and sample handling to compliant data flow and temperature-controlled transport, our services are tailored to help sponsors, CROs, and clinical teams maximise efficiency and maintain full compliance throughout their project lifecycle.
Sponsors
CROs
Clinical teams

Tailored to help sponsors, CROs, and clinical teams
UKBS offers complete support for clinical research and trials, managing everything from sample logistics and storage to compliant transport and kit provisioning.
Our solutions are built to streamline operations, protect sample integrity, and ensure full regulatory compliance across the study lifecycle.
IATA-certified, temperature-controlled transport
End-to-end clinical trial logistics and sample handling
Custom clinical kit assembly and sourcing
Screening and processing via trusted partner labs
Automated and manual storage across all temperature ranges
HTA-licensed biobanking with audit-ready tracking and traceability

Comprehensive Trial Logistics
We take the complexity out of clinical trial logistics. Our team manages everything from collection scheduling to packaging, labelling, and the controlled movement of biological samples. Whether transporting ambient, refrigerated, or frozen material, we offer A-to-B specialist driver services and regulatory-compliant packaging—including IATA-certified dry ice shipping. All drivers are dry ice trained to ensure chain of custody and temperature integrity are maintained from site to lab.
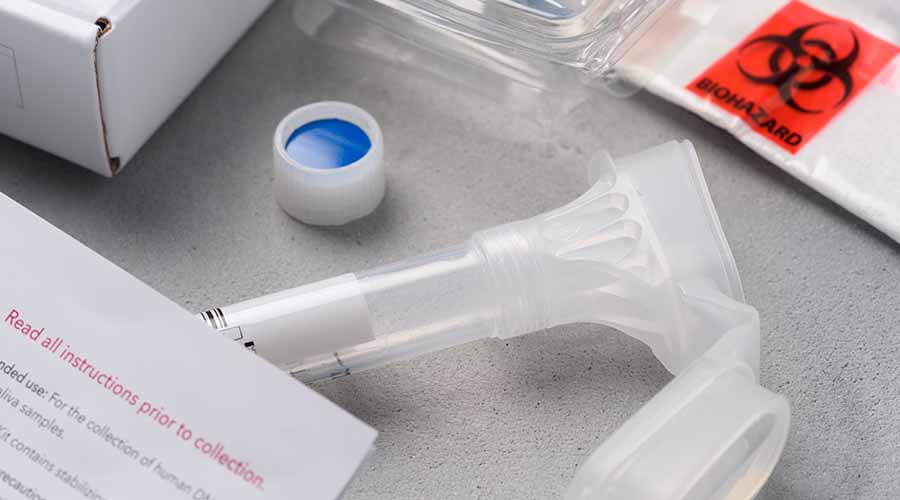
Tailored Clinical Kit Provisioning
We can assist with custom clinical trial kits and supply chain planning. Whether it’s tubes, labels, or transport containers, UKBS sources and assembles the components required for seamless clinical sample collection. This includes ensuring that components are compatible with downstream processes, such as automation-ready racking and 2D barcoded tubes.
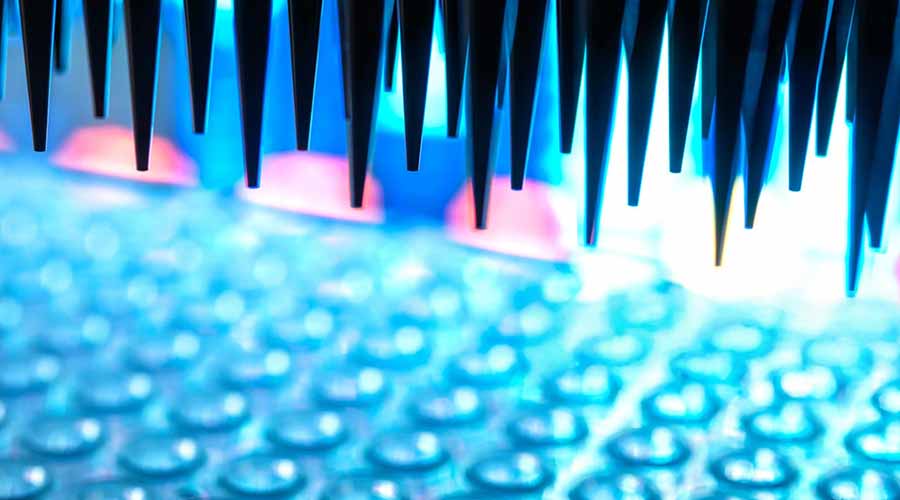
Screening & Sample Processing Support
Working with partner networks including Kaizen Bioservices, UKBS can coordinate and deliver prescreening and screening services tailored to protocol requirements. Services include:
- Sample collection coordination – aligned with inclusion/exclusion criteria
- Sample preparation and aliquoting – minimising freeze-thaw cycles
- Sample analysis – for pharmacogenomics, biomarker, proteomics or safety testing
Where required, Kaizen Bioservices can provide specialist in-house testing capacity or facilitate outsourced testing through accredited partners.
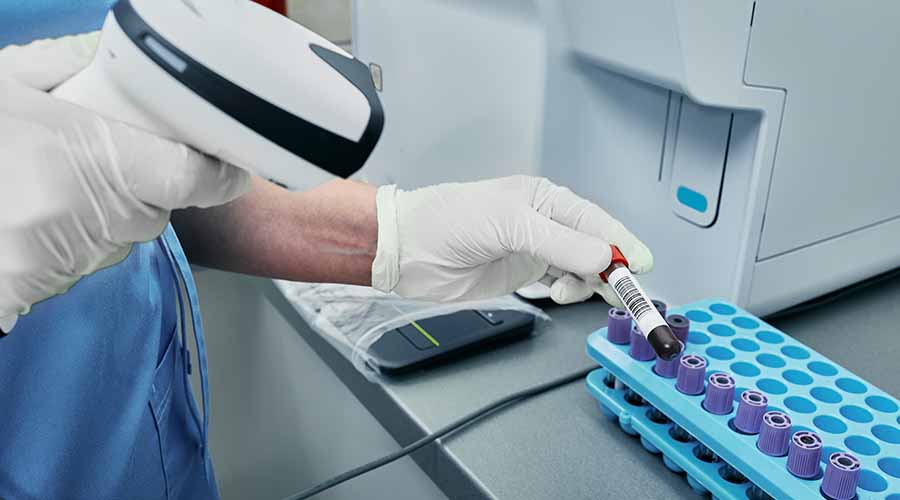
Biobanking & Data-Linked Sample Archiving
Our HTA-licensed biostorage infrastructure supports short- and long-term sample archiving for trials. Samples are catalogued with metadata and stored at room temperature, 2–8°C, –20°C, or –80°C. Fully automated storage systems are available to safeguard irreplaceable material and support future access for longitudinal analysis. UKBS ensures:
Storage compatibility with barcode-labelled tubes and SBS rack formats
Full temperature traceability
Controlled access and audit trails
- Full temperature traceability
- Controlled access and audit trails
- Storage compatibility with barcode-labelled tubes and SBS rack formats
Get in touch
Looking for a reliable, efficient and compliant partner to store your biological samples? Let’s discuss how UK Biostores can support your storage strategy today.
